Technologische Gefahren
Radiological Emergencies
Prepared by TESEC – European Centre of Technological Safety (Kiev, Ukraine)

The nuclear accident at the Chernobyl reactor in 1986 shocked the world: more than 100,000 people in Belarus, Ukraine and Russia were evacuated from the contaminated area and about 5 million were exposed. In France, Germany, Poland and other European countries, radiation protection measures were implemented. The Fukushima nuclear accident in 2011 proved that every nuclear reactor harbours a nuclear hazard.
The public perception of the Chernobyl and Fukushima nuclear accidents clearly demonstrated the lack of public information on radiation hazards associated with releases of radionuclides. As both accidents showed, there is only one trustworthy source of information for communities in such an emergency: their own analysis of the information provided, based on personal basic knowledge.
Nowadays, more than 50% of electricity is produced in Nuclear Power Plants (NPPs) in some countries, and radioactive materials are used in medicine, industry, transport, the military and other areas of human activity. We are exposed to natural radiation from space and the Earth (from granite, thorium sand) or through eating natural radioactive potassium and inhaling radioactive radon. Radiation exposure is part of our lives. On the other hand, there are risks from nuclear or radiological accidents where people can die as a result of radiation exposure.
The term “Radiological Emergency” generally refers to events involving the release of significant levels of radioactivity and the exposure of workers or the general public to radiation as a result of a Nuclear or Radiological Accident. The source of the hazard is ionising radiation – the flux of alpha, beta or gamma particles, which is basically the result of radioactive decay. When the energy of radiation is absorbed by matter, chemical changes occur at the atomic and molecular levels. The amount of radiation energy absorbed per gram of matter is called the absorbed dose. The damage to the tissue from a high dose of radiation is so extensive that the body does not have time to regenerate new tissue, and so the effect becomes visible with many of the features of thermal burning, but it is usually much deeper and longer lasting. Extremely high levels of acute radiation exposure can result in death within a few hours, days or weeks.
Humans are primarily exposed to natural radiation from the sun, cosmic rays and naturally occurring radioactive elements found in rocks, food and the environment. Radon, which emanates from the ground, is another major source of natural radiation. Cosmic rays from space include energetic protons, electrons, gamma rays and x-rays.
The primary radioactive elements found in the Earth’s crust are uranium, thorium and potassium, and their radioactive derivatives. These elements emit alpha and beta particles, or gamma rays.
The average doses of population exposure due to all nuclear industry and man-made radioactive sources amount to about 1% of doses due to natural radiation, but exposure in the event of a nuclear or radiological accident can be far higher and enough to cause death.
Background of Radioactivity. All materials are composed of atoms. An atom consists of a positively charged nucleus and negatively charged electrons, which enclose it. An atomic nucleus consists of positively charged protons and neutrons, which lack a charge. The charge of the nucleus is equal to the number of protons in the nucleus.
The chemical properties of atoms depend on only the number of electrons, equals number of protons in the nucleus. There are atoms with the same chemical properties, but different numbers of neutrons in the nucleus. Consequently, they have different physical properties. Some of these atoms could be unstable, or radioactive.
Radioactivity is the ability of some nuclei to be spontaneously transformed into another nucleus or into the same nucleus with less energy. The extra energy is released by emitting alpha, beta or gamma particles (in special cases neutrons or others particles can be released).
Atoms with the same chemical properties and a different numbers of neutrons in the nucleus are called isotopes or nuclides. The radioactive isotopes are called radionuclides. For example, there are three main isotopes of hydrogen. The light isotope has a nucleus with only one proton and it is stable, so its atomic mass number is A=1, and its symbol is 1H. Deuterium is another isotope of hydrogen; it has a nucleus, which consists of one proton and one neutron, and it is also stable. Its atomic mass number is A=2, and its symbol is 2H. But the isotope of hydrogen, which has a nucleus consisting of one proton and two neutrons, is unstable. Its symbol is 3H and its name is tritium. The stable isotope of iodine is 127I; the isotope 131I (or iodine – 131) is radioactive. Both isotopes have the same chemical properties. The chemical elements in the nature are in some cases the mixture of stable and long-lived isotopes, for example normal potassium, which presents in minerals and food, is mixture of stable isotopes 39K and 41K and long-lived radioactive isotope 40K.
If an isotope is radioactive, what is a unit of radioactivity? One decay per second is a unit of radioactivity called becquerel (symbol Bq). The old unit curie (Ci) equals 3.7×1010 Bq. Different radioactive isotopes (radionuclides) have different rates of decay. The rate of decay is characterized by a half-life, which is the period of time when half of all the radionuclides will decay, transforming to other atoms. Within the half-life, radioactivity reduces two times, after 2 half-live 4 times, after 3 at 8 times, etc. The short-lived radionuclides have a higher radioactivity than the same amounts of long-lived radionuclides. For example, 131I has a half-life of 8.02 days, and cesium-137 (137Cs ) has a half-life of 30.07 years, so 131I has 1370 times higher radioactivity than 137Cs.
Nuclear energy could be released not only as a result of radioactive decay, but also from a nuclear reaction, which occurs when some nuclei interact with others and create new nuclei and could generate energy. Our Sun and other stars shine as a result of nuclear reactions. All existing nuclear power reactors produce energy by a nuclear fission chain reaction, which, unfortunately, produces very high radioactive waste – the main source of radiological hazard. Theoretically, it is possible to create nuclear reactions, which will produce energy without radioactive waste, but for now, nobody knows how to do that. This is a good subject for future research.
A Nuclear Accident is one involving a device using a controlled nuclear chain reaction for some purpose. For example, a Nuclear Reactor has nuclear fuel which, through a self-sustaining and controlled nuclear chain reaction, produces heat, turning turbines and producing electricity. Because of the energy involved in this process, there is potential for considerable radioactive material to be released and dispersed into the environment. Such a release would be due to a “nuclear accident” and results in a Radiological Emergency. Normally, nuclear accidents with releases into the environment are very rare but they have the potential to lead to widespread dispersion of radioactive material.
A Radiological accident is initiated by lost radiation sources, accidents during transportation of radioactive sources or materials, equipment or human errors in the operation of radiation sources. It could result in a Radiological Emergency if there is a risk of human exposure. Sources, often called “sealed sources”, are usually small metal containers in which a small amount of radioactive material is sealed.
Nuclear reactors and radiological accidents. Nuclear reactors are the highest source of radiation and the highest source of radiological risk. As of today, the main way for nuclear energy production is a nuclear fission chain reaction. Heavy nuclei like uranium-235 (235U) splits into two more light nuclei and a few neutrons. Nuclear energy excesses finally transfer to heat energy and then to electricity. The main problem is safety. Nuclei produced in fission are radioactive.
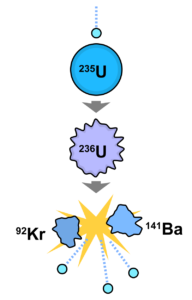
The nuclear
fuel typically used is in the form of uranium dioxide (UO2). The
uranium dioxide is fabricated into cylindrical fuel pellets. These pellets are
stacked end-to-end to form a fuel rod that is encased in fuel cladding.

Each reactor contains a huge amount of radionuclides. If they were distributed to everyone in the World, people would have significant exposure. The main activity in reactors is from fission products, which have different chemical properties – gases like xenon-133 (133Xe), volatile elements like iodine-131 (131I) or cesium-137 (137Cs), or solid materials like strontium- 90 (90Sr) or isotopes of plutonium. The main problem for the safe reactor operation is blocking possible release of radionuclides into the environment. Four barriers prevent the release of radioactive fission products from the reactor core to the environment: fuel pellets, fuel cladding, the reactor vessel, and the containment building. Fuel rods trap 99% of all fission products in the fuel pellets and the remaining 1% in the fuel cladding. If the core is not sufficiently covered with water to provide cooling, it could overheat and cause a breakdown in the fuel cladding, and then a fuel meltdown. Even if the fuel cladding were to fail, two more restraints prevent a release to the atmosphere. The reactor core is located within a reactor vessel (for main types of reactors), that has walls of steel about 30 centimeters thick. The containment building is the last barrier between the radioactive products and the environment (not all types of reactors have a containment building and they have less safety barriers). It is made of high-density, reinforced concrete as much as two meters thick. The containment building is built to withstand severe accidents and natural and technological hazards (like aircraft crash). Even if the first three barriers are damaged, the containment building should prevent any significant release of fission products to the environment.
The average dose of exposure due to all nuclear industry and man-made radioactive sources is about 1% from doses due to natural radiation, but it is not the case of nuclear or radiological accident.
Nuclear or Radiological Accident generally refers to events involving the release of a significant amount of radioactivity from reactor (facility), the exposure of workers and/or the general public to radiation.
Radiological accidents are initiated by the lost radiation sources, accidents during transportation of radioactive sources or materials, equipment or human errors in radiation sources operation. Sources, often called „sealed sources,“ are usually small metal containers in which a small amount of a radioactive material is sealed. Lost source accidents are ones in which a radioactive source is lost, stolen or abandoned. A people finding these sources and not knowing what they are, keep them or even open them and suffer serious exposures.
The nuclear reactors are the most powerful sources of radiation and nuclear accident. If barriers preventing radioactivity release from the reactor core are damaged, first radioactive gases and volatile 131I or 137Cs will be released to the environment. One of the most severe types of accident is a core melt accident. A core melt accident occurs when the heat generated by a nuclear reactor exceeds the heat removed by the cooling systems to the point where at least one nuclear fuel rod exceeds its melting point. A core melt accident can occur even after a reactor is shut down because the fuel continues to produce heat from radionuclides decay. When a nuclear reactor has been shut down and chain nuclear fission is not occurring, a very high source of heat production will still exist due to the radioactive decay of fission fragments. At the moment of reactor shutdown, decay heat will be about 6.5% of the total core power if the reactor has had a long and steady power history. About 1 hour after shutdown, the decay heat will be about 1.5% of the previous core power. After a day, the decay heat falls to 0.4%, and after a week it will be only 0.2%. The decay heat production rate will continue to slowly decrease over time; the decay curve depends upon the proportions of the various fission products in the core and upon their respective half-lives.
The heating of fuel pellets can result in some of the fission products being lost from the pellet. If the radioactive xenon and iodine rapidly leave the pellet, the amount of 134Cs and 137Cs, which is present in the gap between the cladding and the fuel, will increase. If the zircaloy tubes holding the pellets are broken, a greater release of radioactive gases, iodine and cesium from the fuel will occur.
The potential danger from an accident at a nuclear reactor is from exposure to radiation. This exposure could come from the release of radioactive material from the reactor into the atmosphere, usually characterized by a plume (cloud-like) formation. The size of the affected area is determined by the amount and properties of radioactive material released from the reactor, wind direction and speed, and weather conditions (such as rain or snow), which would quickly drive the radioactive material to the ground, causing increased deposition of the radionuclides. Significant contamination could affect areas up to 30 kilometers from the accident site.
The radiation dose received by the public during the first days of a nuclear reactor accident comes mostly from five sources:
1) external gamma radiation from the radioactive cloud or plume, called cloud shine;
2) external gamma radiation from radioactive material deposited on the ground, called ground shine;
3) external beta and gamma radiation from radioactive material deposited on the skin and clothes, buildings and trees;
4) internal exposure from inhaling radioactive material in the plume; and
5) internal exposure from eating and drinking contaminated water and food.
During a release, the exposure dose from cloud shine, ground shine, skin and clothing contamination and inhalation of radioactive material are the most dangerous. After the plume has passed, the dose from ground shine and eating of contaminated food and milk become most dangerous. Doses from external exposure and inhalation can be prevented or reduced by what are referred to as urgent protective measures. These are protective measures that must be implemented urgently or immediately and include sheltering, evacuation, and thyroid blocking. Doses from ingestion can be reduced by restricting immediate consumption of locally produced food.
Nuclear reactors (used for power generation, military or research purposes) are the main sources of radiation. The radioactivity of a nuclear reactor core is millions of times higher than any other man-made source of radiation. Although the construction and operation of nuclear power plants are closely monitored and regulated, an accident, though unlikely, is possible.
The potential danger from an accident at a nuclear reactor is exposure to radiation. This exposure could come from the release of radioactive material from the reactor into the environment, usually characterised by a plume (cloud-like) formation. The size of the area affected is determined by the amount of radioactive material released from the plant, wind direction and speed, and weather conditions (eg rain, snow) which would quickly drive the radioactive material to the ground, causing increased deposition of radionuclides. Significant contamination could affect areas up to 30 kilometres from the accident site.
Radiological accidents can occur wherever radioactive materials are used, stored or transported. In addition to nuclear power plants, hospitals, universities, research laboratories, industries, major highways, railways and shipping yards could be the site of a radiological accident. Radioactive sources are frequently used in industrial gauges (eg moisture and density gauges). If these gauges or other radiation-containing equipment are disposed of incorrectly or sent for recycling as scrap metal, the sealed source may be „lost“ and people may be exposed as a result. They are among the most frequently reported radioactive contaminants in shipments received by scrap metal facilities. If a steel mill melts a source, it contaminates the entire batch of metal, the processing equipment and the facility. More importantly, it can result in the exposure of workers or users to radiation.
There have also been incidents in which unsuspecting individuals find these sources and, not knowing what they are, keep them or even open them and suffer serious exposure. Some satellites use radioactive materials as a power source during long space flights. During the launch or re-entry of satellites there is potential for an accident that would disperse radioactive materials.
Radiation is used in medicine, the military and industry.
The main users of man-made radiation include:
- nuclear reactors and their supporting facilities such as fuel preparation plants;
- medical facilities such as hospitals and pharmaceutical facilities;
- research and teaching institutions;
- facilities involved in nuclear weapons production.
More than 400 Nuclear Power Reactors are in operation around the world (see https://cnpp.iaea.org/pages/index.htm ).
The most serious accident in the history of the nuclear industry occurred on 26 April 1986, at Unit 4 of the Chernobyl nuclear power plant in the former Ukrainian Republic of the Union of Soviet Socialist Republics, near the common borders of Belarus, the Russian Federation and Ukraine. Major releases of radionuclides from the Chernobyl reactor continued for ten days following the explosion on 26 April.
These included radioactive gases, condensed aerosols and fuel particles. The total release of radioactive material was about 14×1018 Bq. More than 200,000 square kilometres of Europe were contaminated with levels of 137Cs above 37 kBq/m2. Much of this area was within the three most affected countries, Belarus, Russia and Ukraine. More than 100,000 people in Belarus, Ukraine and Russia were evacuated from the contaminated area and about 5 million were exposed. In France, Germany, Poland and other European countries, radiation protection measures were implemented.
Acute radiation syndrome due to high exposure (ARS) was diagnosed in 134 emergency workers exposed from 1 to 16 Gy of whole-body irradiation. Twenty eight patients died within three months after exposure. Thyroid cancer in those exposed to 131I at a young age is recognised as a major health effect of the accident confirmed by the findings of many national and international studies. Twenty five years after the accident, nearly 6,000 cases of thyroid cancer have been diagnosed in persons exposed at the age of 0-18 in Belarus, Russia and Ukraine. See more about radiological hazards here .
The Fukushima-1 (Dai-ichi) nuclear accidents were a series of ongoing equipment failures and releases of radioactive materials at the Fukushima-1 Nuclear Power Plant, as a result of the 9.0 magnitude earthquake and tsunami on 11 March 2011. The nuclear plant was flooded by tsunami waves. Evidence soon arose of a partial core meltdown in reactors 1, 2, and 3; hydrogen explosions destroyed the upper cladding of the buildings housing reactors 1, 3, and 4; an explosion damaged the containment inside reactor 2; and multiple fires broke out at reactor 4. Despite being initially shut down, reactors 5 and 6 began to overheat. Fuel rods stored in pools in each reactor building began to overheat, as water levels in the pools dropped.
The total discharge amounts from the reactors of Fukushima-1 NPP were estimated as 0.16 EBq for 131I and 0.015 EBq 137Cs.
Approximately 7,800 emergency workers were exposed to about 7.7 mSv on average. Thirty people were recorded as receiving doses over 100 mSv.
Three workers are reported to have suffered suspected radiation burns on their feet/legs from inadvertent exposure to heavily contaminated water in a turbine basement.
To avert potential radiation exposure to the public, the Japanese authorities took the precautionary measure of instructing those within the first 3 km, then 10 km and finally 20 km of the plant to evacuate, and those between 20 km and 30 km to take shelter and prepare to evacuate. More than 70,000 people have been evacuated since the incident.
The worst commercial accident in the United States occurred at the Three Mile Island nuclear station in 1979. As a result of equipment failures and operator error, a valve that was stuck open allowed coolant water that covered the reactor core to escape from the reactor system for over two hours.
This radioactive water, nearly a million gallons, ended up on the basement floors of the containment building and auxiliary buildings. The loss of coolant water in the reactor core continued to the point that the fuel was no longer submerged in water. Without the cooling provided by the water, the cladding and some of the fuel pellets melted. Large quantities of radioactive material were released into the containment building.
Radiological accidents are initiated by lost radiation sources, accidents during transportation of radioactive sources or materials, equipment or human errors in radiation sources operation.
One of the most severe radiological accidents took place in September 1987 at Goiania, Brazil. A radiotherapy unit had been abandoned in a clinic, which was being demolished. The unit had a source consisting of
5×1013 Bq of cesium-137, sealed within two nested stainless steel containers to form a 5-cm diameter capsule.
Two individuals dismantled the unit and extracted the source, before taking it home and opening it. On 21 September the source material was removed and distributed among several persons, some of whom spread it on their skin. Of the 112,800 or so people who were examined, 129 were found to be contaminated and 9 persons died.
The main adverse consequences of nuclear or radiological accidents are the following:
- Consequences for health: deterministic and stochastic effects
- Psychological consequences
- Environmental consequences
- Social and Economic consequences.
Consequences for health.
There are basically two types of physical health effect related to radiation exposure:
- Deterministic effect
Deterministic effects are the result of acute exposure, which is exposure to a large, single dose of radiation, or a series of doses, over a short period of time. In most cases, large acute exposure to radiation can cause both immediate and delayed effects. For humans and other mammals, acute exposure, if large enough, can cause rapid development of acute radiation sickness (ARS), evidenced by gastrointestinal disorders, bacterial infections, haemorrhaging, anaemia and other issues. Immediate effects occur relatively soon (within days to weeks) after exposure to a high dose at a high dose rate. Essentially, the damage to the tissue from the radiation is so extensive that the body does not have time to regenerate new tissue, and so the effect becomes visible with many of the features of thermal burning, but it is usually much deeper and longer lasting. Deterministic effects often appear localised on the body depending on the radiation exposure pattern and the level of penetration of the radiation. Extremely high levels of acute radiation exposure can result in death within a few hours, days or weeks. Delayed biological effects can include cataracts, temporary sterility, cancer and genetic effects.
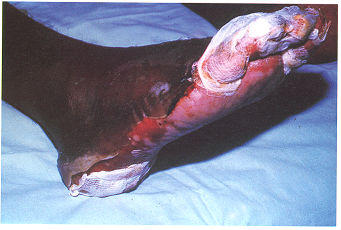
- Stochastic effect
A second type of health effect that can be caused by radiation is a so-called stochastic effect, such as cancer or hereditary effects in any future offspring. These types of effects are characterised by their late appearance after exposure (several years, possibly up to decades) and, critically, by the fact that their occurrence is not certain. The radiation may cause some damage to the cells of the body which is not visible but changes the functioning of those cells. These changes may manifest themselves at a much later date, as a cancer for example. Notice that we say ‘may’ manifest themselves, as there is no certainty of occurrence. For stochastic effects, we find that the chance or probability of an effect increases as the radiation dose increases. So at low doses there is a very low chance of cancer developing – at very high doses, there is a higher chance of cancer. However, it appears that there is no „safe“ dose, or dose threshold below which cancers do not occur. Also it appears that it is the cumulative dose that influences the chance of cancer development and not the dose rate (at least not strongly).
But nuclear and radiological accidents also have consequences other than direct physical effects on humans:
Psychological health effects will always accompany a nuclear or radiological accident whether or not it has resulted in persons receiving significant radiation exposure. Some protective actions taken during Chernobyl to reduce the radiological health risks, such as relocation and resettlement, did more harm than good in some cases because of the resulting psychological health effects brought on by stress and anxiety.
Environmental consequences
When land, water or air becomes contaminated with radioactive material, there is concern about the environmental effects. Normally, radiation does not affect the ecosystem unless the levels are very high, although it can damage individual plants and animals. More problematic is the impact of countermeasures on the environment – countermeasures that were taken to protect humans. Moreover, when the environment becomes contaminated with radioactive material, even if the levels are very small, there is concern among the population continuing to live there. Finally environmental processes, such as wind movement and rivers can transport radioactive material from one place to another, which raises further concerns.
Social and Economic consequences
Any countermeasures taken to address the health or environmental impact will have associated costs, whether the direct cost of the countermeasure itself or the lost economic output from formerly productive areas.
Combining the health and environmental impact with the social consequences associated with the accident and any countermeasures employed, it is clear that the consequences are often more than just the direct health consequences alone.
All practical steps must be taken to prevent and mitigate nuclear or radiological accidents. The most harmful consequences arising from facilities and activities have come from the loss of control over a nuclear reactor core, nuclear chain reaction, radioactive source or another source of radiation.
Consequently, to ensure that the likelihood of an accident having harmful consequences is extremely low, measures have to be taken in order to:
- Prevent the occurrence of failures or abnormal conditions (including security breaches) that could lead to such a loss of control;
- Prevent the escalation of any such failures or abnormal conditions that do occur;
- Prevent the loss of or the loss of control over a radioactive source or another source of radiation.
Preparedness, response and relief are the main tools for minimising the consequences of a Radiological Emergency. The primary goals of preparedness and response for a nuclear or radiological emergency are:
- To ensure that, for reasonably foreseeable incidents, radiation risks would be minor;
- For any incidents that do occur, to take practical measures to mitigate any consequences for human life and health and the environment.
The licensee, the employer, the nuclear regulatory body and appropriate branches of government must establish arrangements, in advance, for preparedness and response for a nuclear or radiation emergency on the spot (on-site plans) and at local, regional and national levels (off-site plans) which, where pertinent, have been agreed at international level.
Relief is usually a gradual process: safety is a primary issue, as are physical and mental well-being.
Many lessons have been learned from the Chernobyl experience in the field of post-crisis administration and rehabilitation:
- Socio-economic recovery is the most significant problem faced by regions affected by the Chernobyl disaster;
- A lack of reliable information led to general mistrust of the authorities and in particular, of official statements on radiation levels;
- Hindrances to effective communication with the public greatly delayed the recovery process itself.
The role of trustworthy information remains important in territorial rehabilitation and providing protection for the population from radiation.
Evacuation and resettling more than a hundred thousand people was justified on grounds of radiation safety but caused psychological stress.
The later resettlement of people from areas of low contamination was not justified: that experience has implications for responding to any future accident.
Anxiety about the health consequences of radiation exposure has not diminished over time. In affected areas some inhabitants are in a state of helplessness and passivity and unable to take decisions about their future. Innovative approaches are needed to involve the affected communities in measures to improve their living conditions in contaminated areas. There is a need to present information to certain groups of persons, who can use it and give helpful advice to the affected population, using an integrated approach to a healthy lifestyle and not only where radiation dangers are concerned.
Ionising radiation affects people by depositing energy in body tissue, which can cause cell damage or cell death. In some cases there may be no effect. In other cases, the cell may survive but become abnormal, either temporarily or permanently, or an abnormal cell may become malignant. Large doses of radiation can cause extensive cellular damage and result in death. With smaller doses, the person or specific irradiated organ(s) may survive, but the cells will be damaged, increasing the chance of cancer. The extent of the damage depends upon the total amount of energy absorbed by body tissue (absorbed dose), the time period and dose rate of exposure and the particular organ(s) exposed.
Evidence of injury from low or moderate doses of radiation may not show up for months or even years. For leukaemia, the minimum time period between the radiation exposure and the appearance of disease (latency period) is 2 years. For solid tumours, the latency period is more than 5 years. The types of effects and their probability of occurrence can depend on whether the exposure occurs over a large part of a person’s lifespan (chronic) or during a very short portion of the lifespan (acute).
Ionising radiation is a source of risk for humans but it has to be used for the benefit of the community.
Radiation measurement. In order to estimate radiological hazard, doses of external exposure, and contamination of soil, water, and food, must be measured. There are a wide variety of instruments used to measure different types of radiation, different energy ranges, and different precision. Here are a few examples. In radiography such as a chest X-ray, the variation of the penetrating power of X-rays in bone and tissue gives rise to an image on photographic film or other device. An ionization chamber collects the charge produced by radiation in a gas. Other instruments measure scintillations in crystals that are produced by radiation.
To measure external exposure from a radioactive cloud or contaminated surface, the dose rate or dose meters are used. For evaluation of internal exposure, we have to know the concentration of different radionuclides like 131I, 137Cs, 90Sr, 239Pu in air, water, and food. Different radionuclides will have different effects upon internal exposure depending on their metabolism in the human organism, and the type of radiation emitting (alpha, beta or gamma). To detect radioactivity, samples of water, and food, etc. are collected, prepared, and measured by a gamma-spectrometer or other instruments. To determine the concentration of radionuclides in the air, it is pumped through filters and concentrations of radionuclides are measured.


The primary means of accident prevention is „defence in depth“. Defence in depth is implemented primarily through a combined number of consecutive and independent levels of protection that would have to fail before harmful effects could be caused to people or to the environment. If one level of protection or barrier were to fail, the subsequent level or barrier would be available. When properly implemented, defence in depth ensures that no single technical, human or organisational failure could lead to harmful effects, and that the combinations of failures that could trigger significant harmful effects are of very low probability. The independent effectiveness of the different levels of defence is a requisite component of defence in depth.
Defence in depth is provided by an appropriate combination of:
- An effective management system with a strong management commitment to safety and a strong safety culture.
- Adequate site selection and the incorporation of good design and engineering features providing safety margins, diversity and redundancy, mainly by the use of:
- Design, technology and materials of high quality and reliability;
- Control, limiting and protection systems and surveillance features;
- An appropriate combination of inherent and engineered safety features;
- Comprehensive operational procedures and practices as well as accident management procedures.
Mitigation corresponds to taking measures to limit the adverse impact of a Nuclear or Radiological Accident and it is based on two main components:
- Planning for emergencies on the site of a hazardous facility and off-site;
- Community awareness.
The practical goals, following on-site and off-site emergency planning, are to:
- Regain control of the situation;
- Prevent or mitigate consequences at the scene;
- Prevent the occurrence of deterministic health effects in workers and the public;
- Give first aid and manage the treatment of radiation injuries;
- Prevent, to the extent practicable, the occurrence of stochastic health effects in the population;
- Prevent, to the extent practicable, the occurrence of adverse non-radiological effects on individuals and among the population;
- Protect, to the extent practicable, the environment and property; and
- Prepare, to the extent practicable, for the resumption of normal social and economic activity.
There are three general rules for controlling exposure to ionising radiation in the event of a Radiological Emergency:
- minimising exposure time;
- maximising distance from the radiation source;
- protecting oneself from the radiation source.
Time is an important factor in limiting exposure for the public and for radiological emergency responders. The shorter the period of time an individual stays in a radiation field, the smaller the dose they will receive.
Consequently, the strategy for reducing public risk in the most severe reactor core damage accidents is the following:
Before or shortly after radioactive release – based on plant conditions
- Evacuate or take substantial shelter within 3-5 km.
- Take iodine prophylaxis near the plant.
After a release
- Carry out prompt monitoring to locate areas requiring further protective actions.
- Restrict consumption of locally grown food up to 300 km, following monitoring results.
- Undertake monitoring to locate where food restrictions and relocation are warranted.
The emergency plans for facilities with the highest radiological risk (such as nuclear power reactors) define two emergency planning areas:
ON-SITE AREA
This is the area surrounding the facility within the security perimeter, fence or other designated property marker. It can also be the controlled area around a radiation source or contaminated area. It is the area under the immediate control of the facility or operator. For transport emergencies or emergencies involving uncontrolled sources or localised contamination, there may not be an on-site area defined at the onset of the emergency.
OFF-SITE AREA
This is the area beyond the on-site area. For facilities with the potential for emergencies resulting in major off-site releases or exposure, the level of planning will vary depending on the distance from the facility. For these facilities, three emergency planning zones can be defined:
Precautionary action zone (PAZ): 3- 5 km from the radiation source
This is a predefined area around a facility, where urgent protective action has been planned and will be implemented immediately upon declaration of a general emergency. The goal is to substantially reduce the risk of severe deterministic health effects by taking protective action within this zone before or shortly after a release.
Urgent protective action planning zone (UPZ): 25 km from the radiation source
This is a predefined area around a facility where preparations are made to promptly implement urgent protective action based on environmental monitoring data and assessment of facility conditions, the goal being to avert doses specified in international standards.
Food restriction planning radius (FRR): 300 km from the radiation source
This is the area where preparations are made for effective implementation of protective actions to reduce the risk of stochastic health effects from the ingestion of contaminated locally grown food. In general, protective actions such as relocation, food restrictions and agricultural countermeasures will be based on environmental monitoring and food sampling.
These zones should be roughly circular areas around the facility, with their boundaries defined by local landmarks (eg roads or rivers) to allow easy identification during response. It is important to note that these zones do not stop at national borders.
The size of the zones can be determined by an analysis of the potential consequences: the generic zone sizes as stated above are based on previous studies (Method for Developing Arrangements for Response to a Nuclear or Radiological Emergency, EPR-METHOD (2003), IAEA, VIENNA, 2003, ISBN 92–0–111503–2).